
At this point in your study of chemistry, you should memorize the names, formulas, and charges of the most common polyatomic ions. Oxyanions are polyatomic ions that contain one or more oxygen atoms. How are non-metal ions formed Non-metal ions are formed when a non. Note that these transactions can normally only take place simultaneously: in order for a sodium atom to lose an electron, it must be in the presence of a suitable recipient like a chlorine atom.\). Metal ions are positive because when the metal atom reacts it looses electrons. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium cation) or –1 (chloride anion) charge. Both ions now satisfy the octet rule and have complete outermost shells. Z Charges on the positive and negative ions r Inter ionic distance A Madelung constant n Born exponent Lattice energies of alkali metal halides. thus limiting the negative consequences on the environment. In this example, sodium will donate its one electron to empty its shell, and chlorine will accept that electron to fill its shell. The removal of metal ions by biosorption on inexpensive materials is still a challenge for. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell.
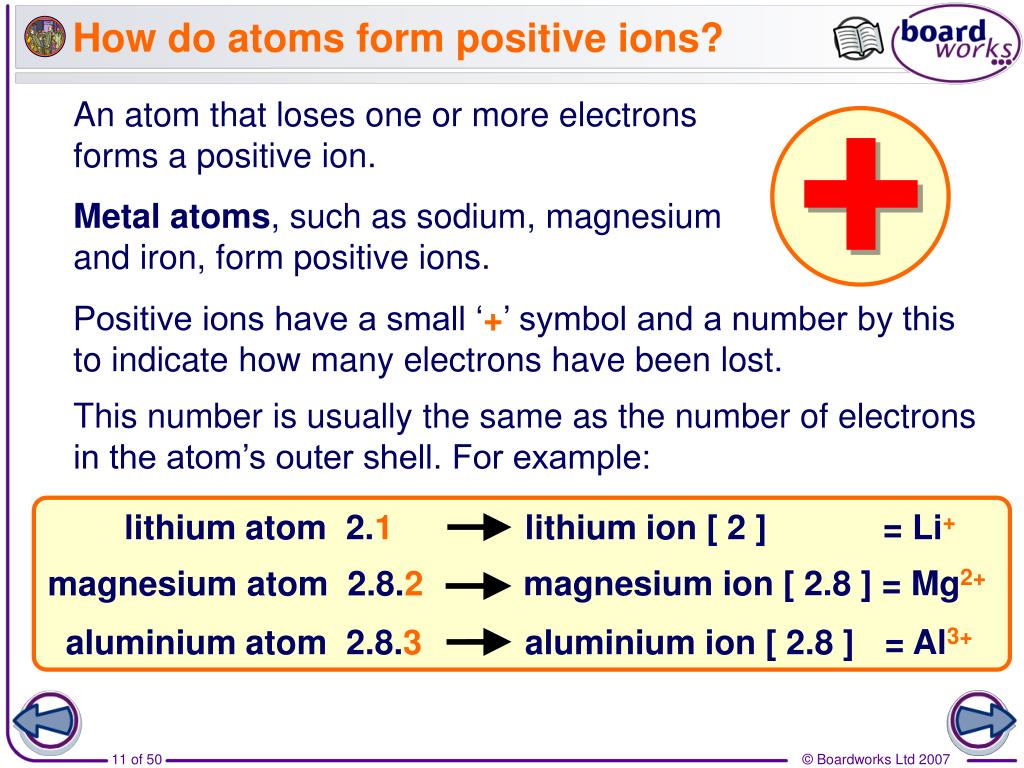
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. Thus, metals are known to have lower electron affinities. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. Remember that ions are formed only when electrons move from one atom to another a proton never moves from one atom to another. As Figure 1 illustrates, sodium (Na) only has one electron in its outer electron shell. Video Test 1 2 3 4 Forming ions An ion is an atom or group of atoms with a positive or negative charge. This movement of electrons from one element to another is referred to as electron transfer. Anions are designated by their elemental name being altered to end in “-ide”: the anion of chlorine is called chloride, and the anion of sulfur is called sulfide, for example. Negative ions are formed by gaining electrons and are called anions. Cations are positive ions that are formed by losing electrons. Because the number of electrons does not equal the number of protons, each ion has a net charge. This fills their outermost electron shell and makes them energetically more stable. When writing the formula of an ionic compound we use the lowest whole number ratio of cations ( + ions) to anions ( - ions).

The strong attraction between positive and negative ions often produce crystalline solids that have high melting points.
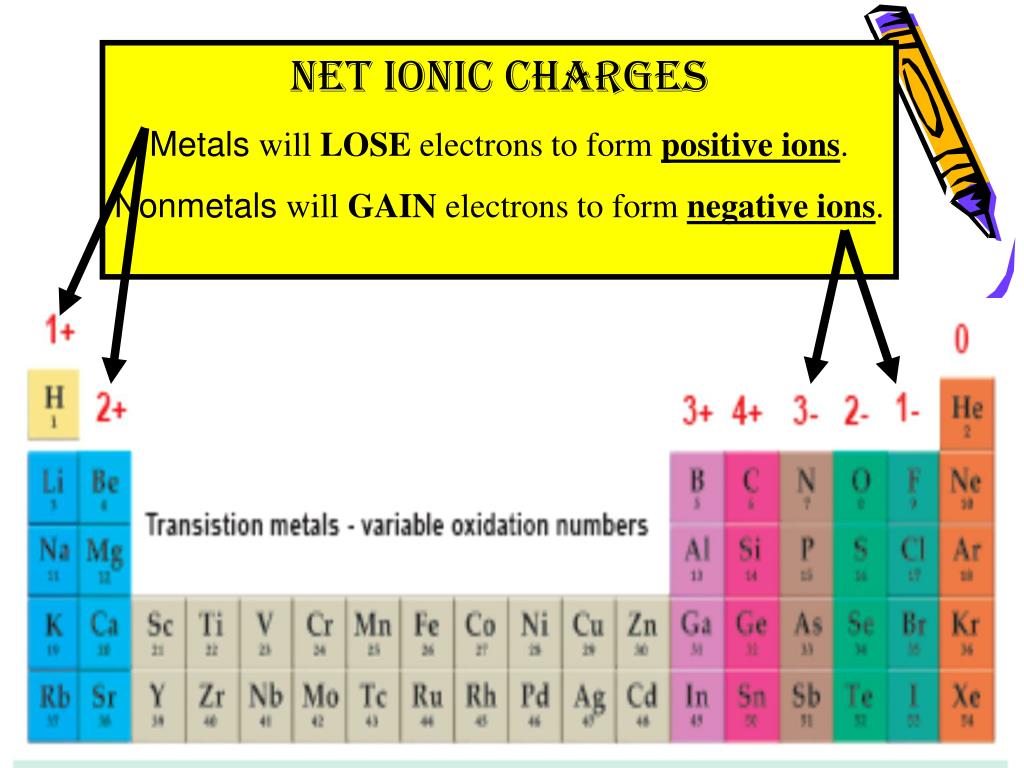
When it comes to generating negative ions, both metal and nonmetal elements can do the job. Updated on JanuIonic compounds form when positive and negative ions share electrons and form an ionic bond.

Non-metals can also form positive ions, but this is much less common. Some atoms are more stable when they gain or lose an electron (or possibly two) and form ions. This is because metals generally have low electronegativity values, meaning that they don’t hold on to their electrons very tightly.


 0 kommentar(er)
0 kommentar(er)
